ElectroChemica 001
In June I began my experiments growing metal dentrites.
The first steps involved determining the appropriate positive and negative connections (anode vs cathode) the appropriate solutions (eg ferrous sulphate, copper sulphate) and which metals worked best from a selection of copper, aluminium and iron - referencing the original electrochemical glass experiment I made in 1997.
 Test set up with variable power supply and 6 cells of all Fe, Cu, Al, + - combinations
Test set up with variable power supply and 6 cells of all Fe, Cu, Al, + - combinations
 one minute 20v
one minute 20v
 10 minutes, strongest reaction occuring between aluminium and copper (lower right), least reaction with aluminium as + anode (centre cells).
10 minutes, strongest reaction occuring between aluminium and copper (lower right), least reaction with aluminium as + anode (centre cells).
 Underside of cells, dendrite growth can be seen between Al- Cu+ cell (top right)
Underside of cells, dendrite growth can be seen between Al- Cu+ cell (top right)
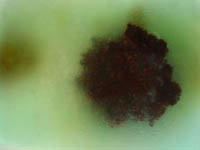 Close up of Al- Cu+ cell
Close up of Al- Cu+ cell
The first steps involved determining the appropriate positive and negative connections (anode vs cathode) the appropriate solutions (eg ferrous sulphate, copper sulphate) and which metals worked best from a selection of copper, aluminium and iron - referencing the original electrochemical glass experiment I made in 1997.
 Test set up with variable power supply and 6 cells of all Fe, Cu, Al, + - combinations
Test set up with variable power supply and 6 cells of all Fe, Cu, Al, + - combinations one minute 20v
one minute 20v 10 minutes, strongest reaction occuring between aluminium and copper (lower right), least reaction with aluminium as + anode (centre cells).
10 minutes, strongest reaction occuring between aluminium and copper (lower right), least reaction with aluminium as + anode (centre cells). Underside of cells, dendrite growth can be seen between Al- Cu+ cell (top right)
Underside of cells, dendrite growth can be seen between Al- Cu+ cell (top right) Close up of Al- Cu+ cell
Close up of Al- Cu+ cell
0 Comments:
Post a Comment
<< Home